TRAM Live-cel Technology
TRAMs - T-cell rapamycin attenuated micropharmacies (PMID 35298086). Rapamycin treated T-cells are genetically engineered to provide durable cross-correction, which was recently demonstrated in cellular models for Fabry, Gaucher, Farber, and Pompe Disease.
Fabry TRAM Live-cel Program (GLA: alpha-galactosidase A):
Treating Fabry disease caused by GLA deficiency - a condition affecting over 800,000 persons worldwide.
Pompe TRAM Live-cel Program (GAA: acid maltase):
Treating Pompe disease caused by GAA deficiency - a condition affecting over 400,000 persons worldwide.
Gaucher TRAM Live-cel Program (GBA1: acid glucosylceramidase):
Treating Gaucher disease caused by GBA1 deficiency - a condition affecting over 160,000 persons worldwide.
TRAM Live-cel Therapies
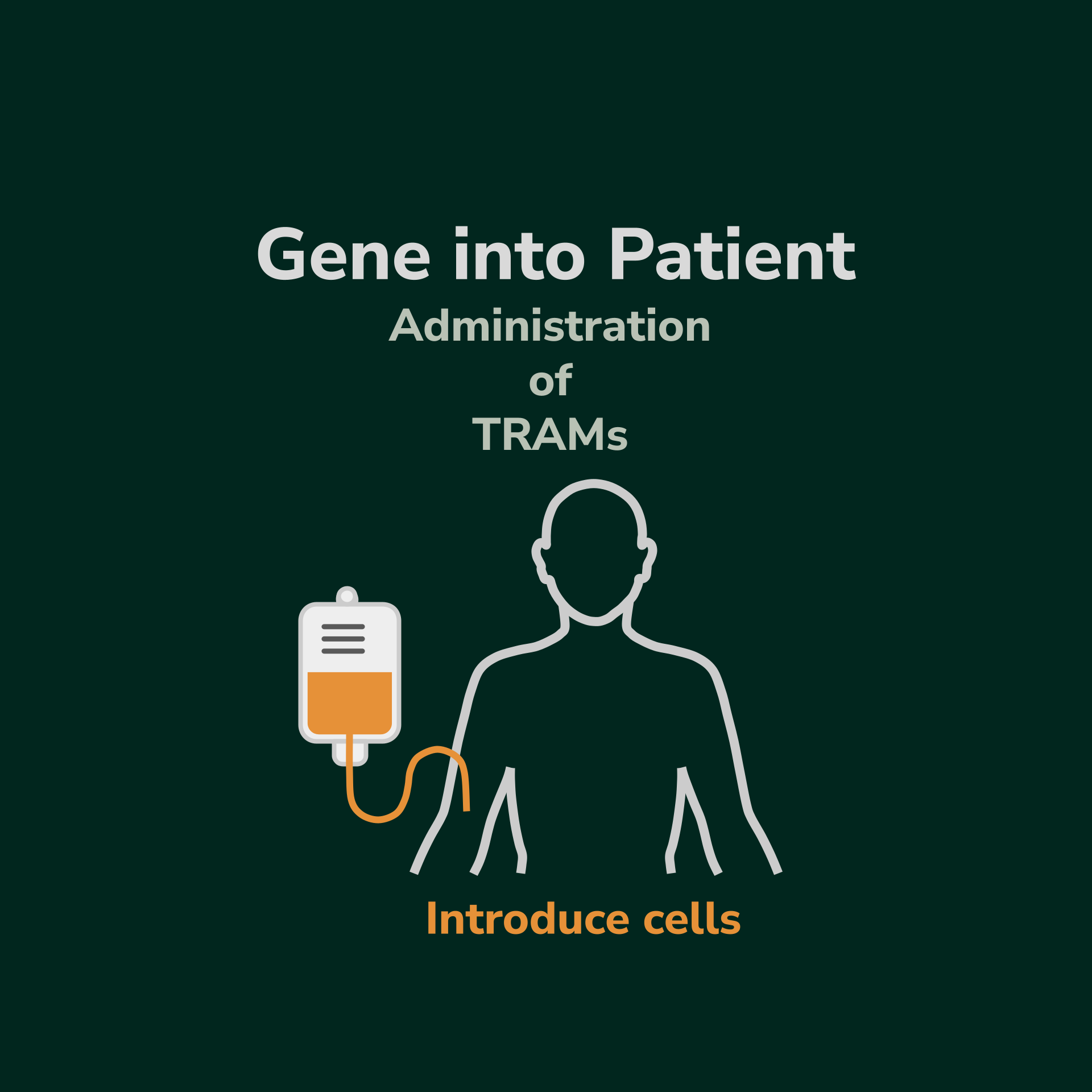
Our Programs using TRAM Live-cel technology are built on our STEM Live-cel technology. Like the STEM method, TRAM Live-cel therapies provide a cell-based gene therapy by using tissue harvested from the patient (autologous-derived). However, unlike STEM, there is no need to mobilize stem cells from the bone marrow. Instead, we use simple apheresis to harvest white blood cells from the patient’s peripheral blood, which is then taken to the lab to isolate the T-cell population. The T cells are manipulated to behave like T-reg cells and express the missing enzyme of interest. The cells are then reintroduced back into the patient where they can then start providing therapeutic amounts of enzyme replacement in the patient.
The TRAM Live-cel procedure involves harvesting the patient’s lymphocytes by aphaeresis.
T-cell populations are isolated in the GMP lab and treated for rapamycin to make T-rapa cells.
T-rapa cells are transduced with lentivector to create a modified, attenuated T-cell (the TRAMs).
The TRAM population is transfused back into the patient where they systemically spread and perdure.
The TRAM Live-cel therapy offers multiple benefits for patients. In addition to providing cross-correction by continuously secreting the replacement enzyme as seen in our STEM Live-cel therapy, studies in animal models and humans suggest rapamycin exposure to T-cells promotes the formation of T-regs, which are critical for keeping autoimmune inflammatory responses systemically attenuated (PMID 31874182). As a result, cellular therapy with TRAMs is expected to have the potential to activate multiple synergistic mechanisms and mitigate the debilitating effect of a variety of genetic disorders.