Glafabra is bringing to market our Live-cel therapy. Our lead asset is Clinical Stage for treating Fabry Disease. The therapy is long acting. Just one dose can lasts for years, not weeks.
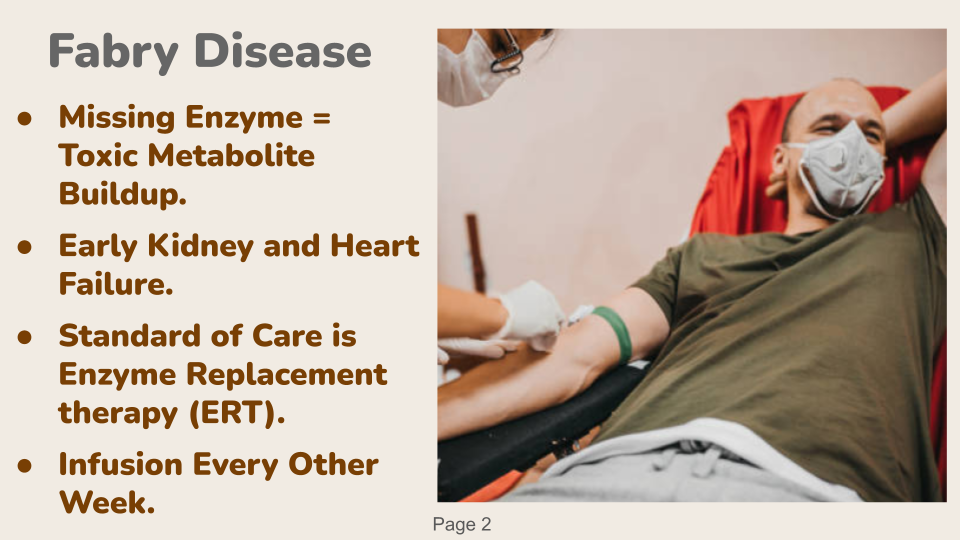
Patient’s with Fabry diseases struggle with the build up of toxic metabolites in their body because they are missing a key enzyme. Without this enzyme, many patients are fated to have kidney failure and heart failure. Most patients are on the standard of care which is enzyme replacement therapy or ERT for short. ERT is tedious on patients becasue they need a protein infusion every other weeks and must build their lives around tedious clinical visits. Fabry affects 400,000 persons arround the world. They need a therapy that lasts for years, not weeks.
Glafabra’s solution for Fabry patients is our Live-cel therapy. They gets a cell-based gene therapy that they can take, one time and get an effect that last for 5 years. With Live-cel treatment patients get the freedom they needs to start living a more normal life.
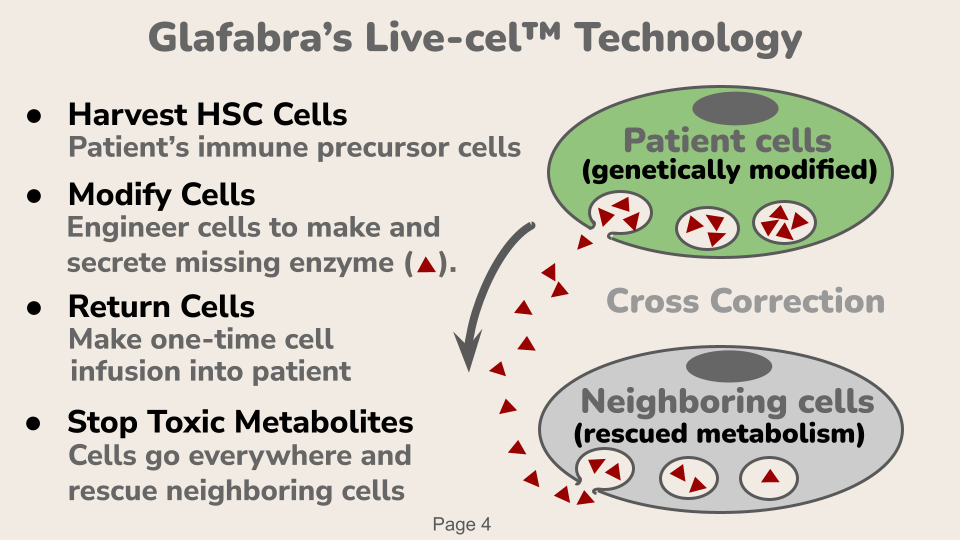
Glafabra’s solution for Fabry patients is a cell-based gene therapy, which occurs in 4 steps:
Harvest Cells. Hematopoietic stem cells are recovered from the patient.
Modify Cells. Recovered cells are treated with lentivector containing the gene for the missing enzyme.
Return Cells. Modified cells are infused back into paitient where they engraft in bone marrow and their progeny produce white blood cells that go everywhere in the body and secrete the missing enzyme.
Control Toxic Metabolites. Neighboring cells take up the secreted enzyme and use it to control the build up of toxic metabolites.
With a single dose of treatment, Patients can start feeling healthy again. This effect will last for many years, from just one dose.
This therapy has been tested in the clinic. We looked the build up of toxic metabolites in patients to measure the effectiveness of our therapy. Initially all the patients were all on ERT. When they were taken off ERT, their toxic metabolites by spiked up 41%. Then, when given the cell therapy, the toxic metabolites dropped 48%. To a level past the starting place. And 5 years later the toxic metabolites were still in check, after just that first initial dose.
The trial with Fabry patients was started in 2016 and completed in 2023. Multiple peer-reviewed publications were achieved. The key ones indicated the therapy is safe, effective and durable.
The therapy has IP coverage. We have an Exclusive Options agreement signed with Medical College of Wisconsin for a patent pending covering composition of mater and methods of use with our transfer plasmid. We are preparing documentation for submission of an Orphan Drug Designation (ODD) with the FDA which would allow us to plan on 7 years of exclusivity at market. We also have a variety of internal and external IP to pursue to enable improved drug delivery.
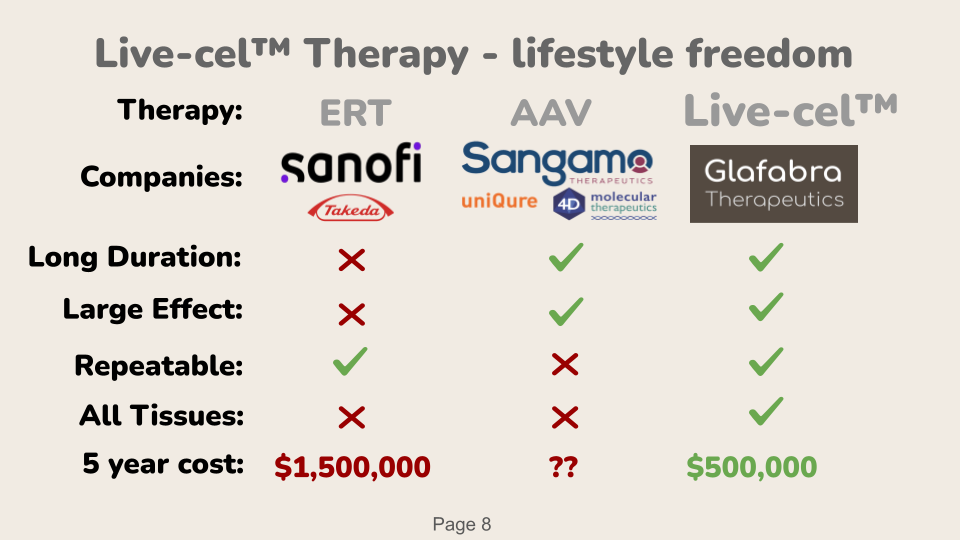
Live-cel treatment gives patients a freedom of lifestyle. They get a Long Duration, from just one dose. Patients get a Large Effect, which allowed patients to have felt the best they has felt in their life. Yet, Patients have alternatives. They could use the emerging technology of AAV, which stands for Adeno Associated Virus. It is also has long duration and a large effect. However AAV is not repeatable, so when the therapy wears off after a few years, patients will need to seek an alternative, such as our Live-cel therapy. Patients can to say “I has felt the best I has felt in my life” because our therapy can reach nearly all tissues, even get past the blood brain barrier, which is very difficult for AAV and ERT to accomplish. Finally, We plan to price our therapy at a cost that is lower than the existing standard of care.
Glafabra’s clinical plan includes treating Fabry, Pompe, and Gaucher Diseases. The combined patient population near 1.3 million. We know there is good money to be made in taking market share - last year Sanofi made nearly $3 B from these 3 disease categories.
We are currently raising 0.5 million in pre-seed funding to help bring our Fabry asset towards an IND filing. However we will need close to 50 million to get all of our programs into the clinic and creating efficacy data which will be needed to enable us to achieve a good exit.
Glafabra has a seasoned team. Myself, Chris Hopkins as CEO. I founded Knudra Transgenics and led its acquisition into InVivo Biosystems. Joining us is Elizabeth Wagner as our COO. She too was involved in the merger of Tarix into Constant Therapeutics. We also have Krista Casazza as director of regulatory science. She comes to us from the critical path institute. We are backed by two co-founders, Doctors Jeffrey Medin and Ronan Foley, who are long-time pioneers in development of our Live-cel therapy approach. We have a variety of advisors in both business and science to help fill in any gaps. Combined, the team has the track record needed to help us succeed.
Glafabra’s Use of Funds with a $500,000 raise will be to obtain an Orphan Drug Designation (ODD), then conduct an INTERACT meeting with the FDA, and get vector configurations updated.
In summary, Glafabra is introducing Live-cel technology. Were creating cell therapies to patients with Fabry. Pompe. And Gaucher. We are in the clinic with our lead asset in Fabry, where it has been demonstrated to be Safe. Effective. And Durable. Our ask is for $0.5 M in pre-seed funding to help us move forward on repeating the Fabry clinical studies in the US. I would like to end on another quote from a patient. “At one point, my wife and I realized we were forgetting I had Fabry at all.”